ELN for Research and Development
Designed to assist researchers and developers in quickly documenting their daily work and meeting regulatory requirements.
Last updated
Designed to assist researchers and developers in quickly documenting their daily work and meeting regulatory requirements.
Last updated
Research and development typically focuses on innovations and proof of concepts, often using a small set of samples. As long as the data generated by researchers can be replicated by other scientists and meets all regulatory requirements, there is no need to document every detail. Scientists prefer to capture results quickly and with minimal effort, which is why they tend to document their data in one segment each day—simply noting what they worked on that day. A rich text editor with support for different file types may be best suited for this purpose.
To use the ELN for Research and Development application, users need to install the "Electronic Lab Notebook (ELN)" application, which will create the necessary "entry" and "protocol" tables.
To install the application:
Select Applications from Settings -> Applications
Click Add application and select Add from a template
Choose Electronic Lab Notebook from the pop-out modal. A lack of such an option indicates that you have already installed it.
"entry" table:
Description: An entry describing an experiment or experiments conducted in a day.
Columns:
"signature_status": Tracks the signature status of the entry.
"protocol" table:
Description: Protocols are procedures for specific experiments or analyses.
Columns:
"status": Tracks the status of the protocol (Drafting, Pending Review, Finalized, Archived).
Sections:
"Steps": Allows users to input the steps of the protocol.
"Steps (Text)": Provides a rich text editor (CKEditor Classic) for inputting protocol details.
"Documents (Attached Files)": Allows users to attach files related to the protocol.
Creating new records:
Users can create new experiment entries by accessing the "entry" table and adding relevant details.
Protocols can be created by accessing the "protocol" table and inputting the required information and steps.
Managing records in table list view:
Users can view and manage existing entries and protocols in the table list view.
Editing, deleting, and organizing records can be done from this view.
Viewing record details:
Users can access the detail view of an entry or protocol to view and edit the specific details, sections, and attachments.
At Labii, we developed the function to make it easy for researchers and developers to quickly document what they worked on each day, as well as meet the requirements of an electronic lab notebook. With this feature, you can easily create quick notes and fulfill all of your research and development needs.
To update an entry's name and description, please refer to the
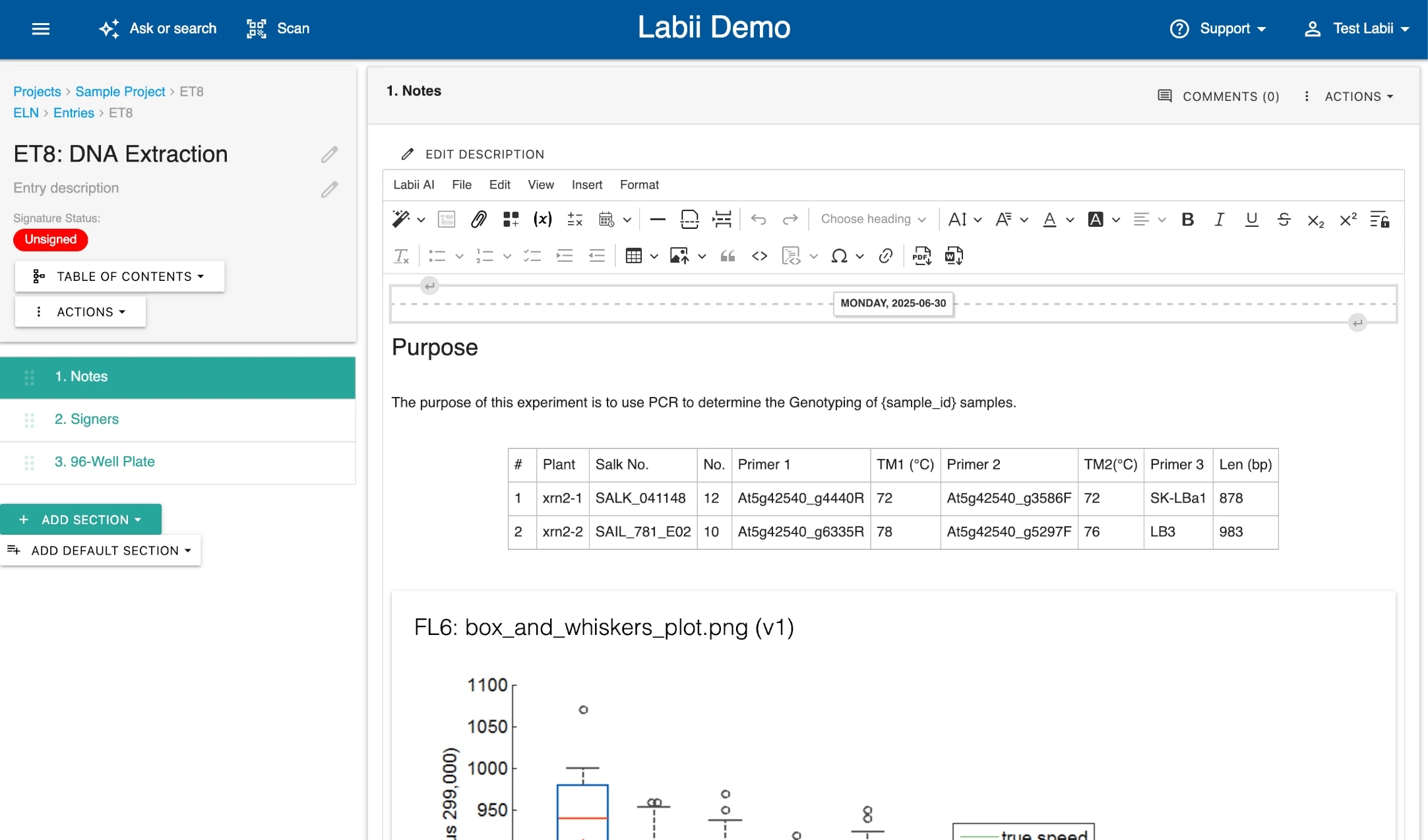
The tab allows you to document research data in rich text. For more information, please refer to the .
By signing a document in , you are certifying that the record you generated or witnessed is accurate to the best of your knowledge. This signature ensures that the data generated meets regulatory requirements, such as FDA 21 CFR part 11 as well as enables other scientists to replicate the results based on the signed document. With a lab notebook containing signatures, you have a legally valid and permanently written record of your lab's research that can be used in a court of law for patent investigations.
Learn more about signing and witness at .
The feature in Labii ELN allows for the tracking and recording of all changes made to an experiment, including who made the changes and when. This feature provides a clear and complete history of an experiment, ensuring that all data is secure and tamper-proof, and easier to identify any potential problems or issues.
You can also print the whole experiment to share. Learn more at .
application offers researchers and developers a convenient and efficient way to document their daily work and meet regulatory requirements. With its user-friendly interface, support for rich text and various file types, and features like signing, audit trail, and version control, the application streamlines the research and development documentation process and ensures accurate and secure records.