ELN for ELISA Data Analysis
ELN tailored for efficient ELISA data analysis, featuring an intuitive ELISA Standard Curve widget meticulously crafted to simplify and expedite the analysis process.
Overview
ELISA (Enzyme-Linked Immunosorbent Assay) is a widely used analytical biochemistry technique for detecting and quantifying substances such as proteins, peptides, antibodies, and hormones. The Electronic Lab Notebook (ELN) for ELISA provides a specialized platform designed to streamline ELISA data documentation, analysis, and interpretation. With the integrated ELISA Standard Curve widget, researchers can efficiently set up plate layouts, document absorbance data, generate standard curves, calculate sample concentrations, and assess assay quality metrics—all within a single, cohesive electronic notebook environment.
This application eliminates the need for multiple disconnected software tools by integrating the entire ELISA workflow from experimental setup through data analysis and electronic signature. Researchers can quickly analyze standard curves, predict concentrations with dilution factor corrections, and interpret results with confidence, while maintaining complete audit trails and regulatory compliance for quality control and research applications.
This application is ideal for immunology labs, diagnostic testing facilities, pharmaceutical research, and any laboratory conducting routine or research ELISA assays requiring efficient data analysis and compliant documentation.
Use Cases
Immunology Research
Quantify cytokine concentrations in cell culture supernatants
Measure antibody titers in serum samples
Analyze immune responses in experimental studies
Compare treatment groups with standardized assays
Document longitudinal studies with consistent methodology
Drug Discovery and Development
Screen compound effects on protein expression
Measure biomarker concentrations in preclinical studies
Conduct dose-response analyses with standard curves
Validate assay performance and reproducibility
Support IND applications with compliant documentation
Clinical Diagnostics
Quantify disease biomarkers in patient samples
Perform routine diagnostic testing with standardized procedures
Track quality control metrics across assay runs
Maintain audit trails for regulatory compliance
Generate reports for clinical decision support
Quality Control Testing
Monitor product quality in biopharmaceutical manufacturing
Validate assay performance with control samples
Track inter-assay and intra-assay variability
Document equipment qualification and calibration
Support GMP compliance requirements
Academic Teaching Laboratories
Teach ELISA principles and techniques
Document student experimental results
Compare results across laboratory sections
Demonstrate data analysis workflows
Create reusable protocols for teaching
Food Safety Testing
Detect allergens in food products
Quantify toxins and contaminants
Perform routine safety screening
Maintain compliance with food safety regulations
Track batch testing results
Getting Started
Navigate to Settings → Applications from the main menu
Click Add application and select Add from a template
Choose Electronic Lab Notebook (ELN) for ELISA from the template list
If this option is not available, the application is already installed in your account.
Wait for the installation to complete. The system will automatically create:
elisa table for ELISA experiments and data analysis
protocol table for standardized ELISA procedures
Access the newly created tables from the side menu to begin documenting ELISA experiments
Application Structure
ELISA Table
The elisa table is designed for documenting complete ELISA experiments from setup through analysis.
Purpose: Record ELISA experiments with integrated plate layout, data entry, and automated standard curve analysis
Key Columns:
date_start: Experiment start date (required)
date_due: Expected completion date for planning
signature_status: Tracks signature workflow (Unsigned, Signed, Witnessed)
Key Sections:
ELISA Workflow: Interactive guide for completing ELISA experiments step-by-step
Experiment Overview: Purpose, hypothesis, and experimental design
Experiment Procedure: Step-by-step protocol documentation
ELISA Standard Curve: Specialized widget for plate layout, data entry, and analysis
Other Files: Supporting documents and raw data files
Experiment Signers: Electronic signature management
Typical Use: Protein quantification assays, antibody detection, biomarker analysis, immunoassays
Protocol Table
The protocol table manages standardized ELISA procedures and validated methods.
Purpose: Create and maintain reusable ELISA protocols and SOPs
Key Columns:
status: Tracks protocol lifecycle (Drafting, Pending Review, Finalized, Archived)
Key Sections:
Steps: Structured step-by-step instructions
Steps (Text): Rich text editor for detailed procedures
Documents (Attached Files): Method specifications, kit inserts, validation reports
Typical Use: Standard ELISA protocols, validated analytical methods, kit-based procedures
Creating a New ELISA Experiment
Navigate to the elisa table from the side menu or search for "elisa"
Click the + Add button at the top of the table list view
Fill in the experiment details:
Name: Descriptive experiment title (e.g., "IL-6 Quantification in Plasma Samples")
Date Start: Experiment start date (required)
Date Due: Expected completion date
Project: Link to relevant project (optional)
Click Save to create the experiment. The detail view opens with the ELISA Workflow section visible
Follow the guided workflow to complete your ELISA experiment systematically
Following the ELISA Experiment Workflow
The ELISA Workflow section provides a structured guide for completing ELISA experiments from setup through analysis and signature.
<fiUnderstanding the ELISA Standard Curve Widget
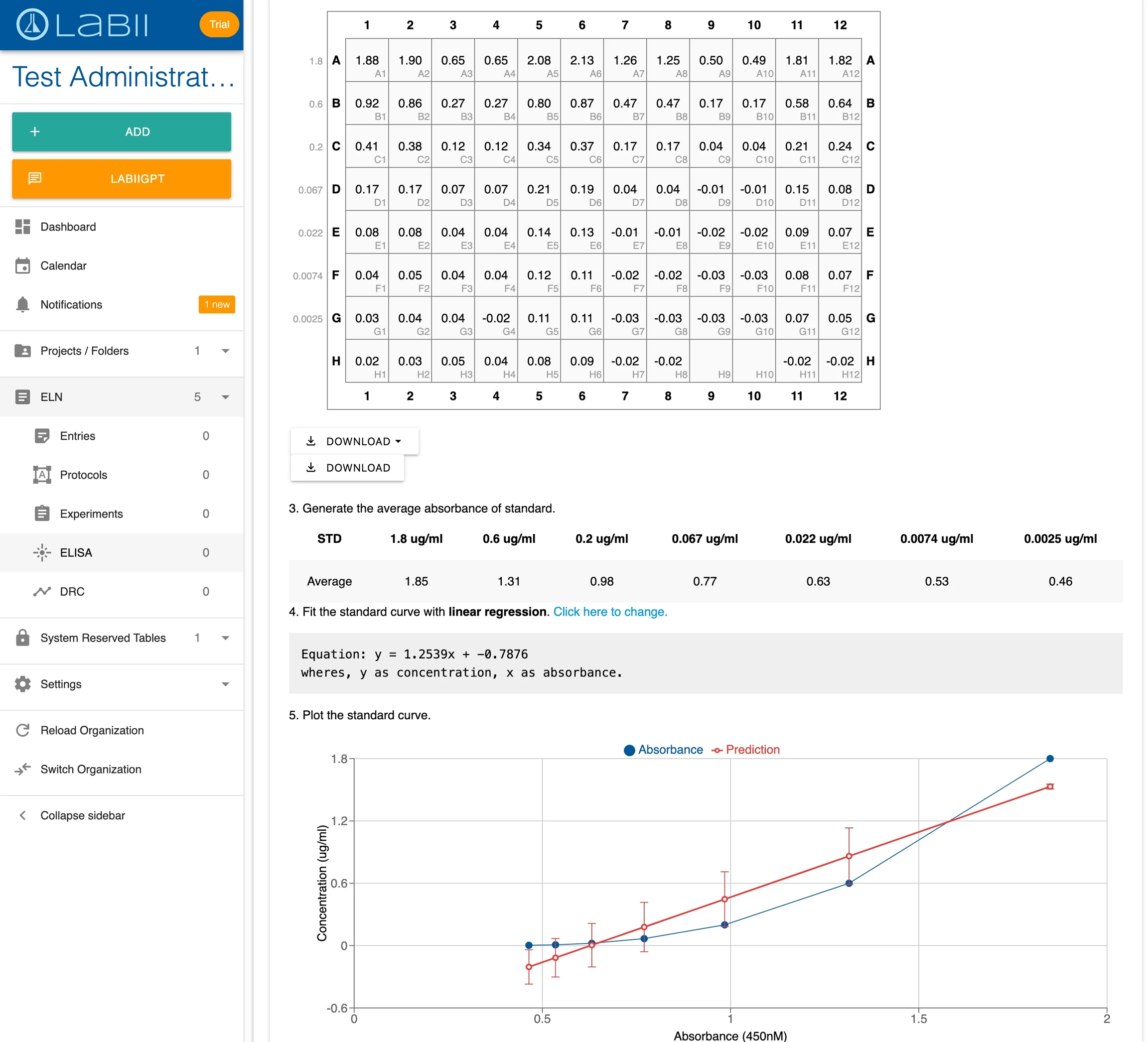
The ELISA Standard Curve widget is a specialized section widget that integrates plate layout, data entry, and automated analysis into a single interface.
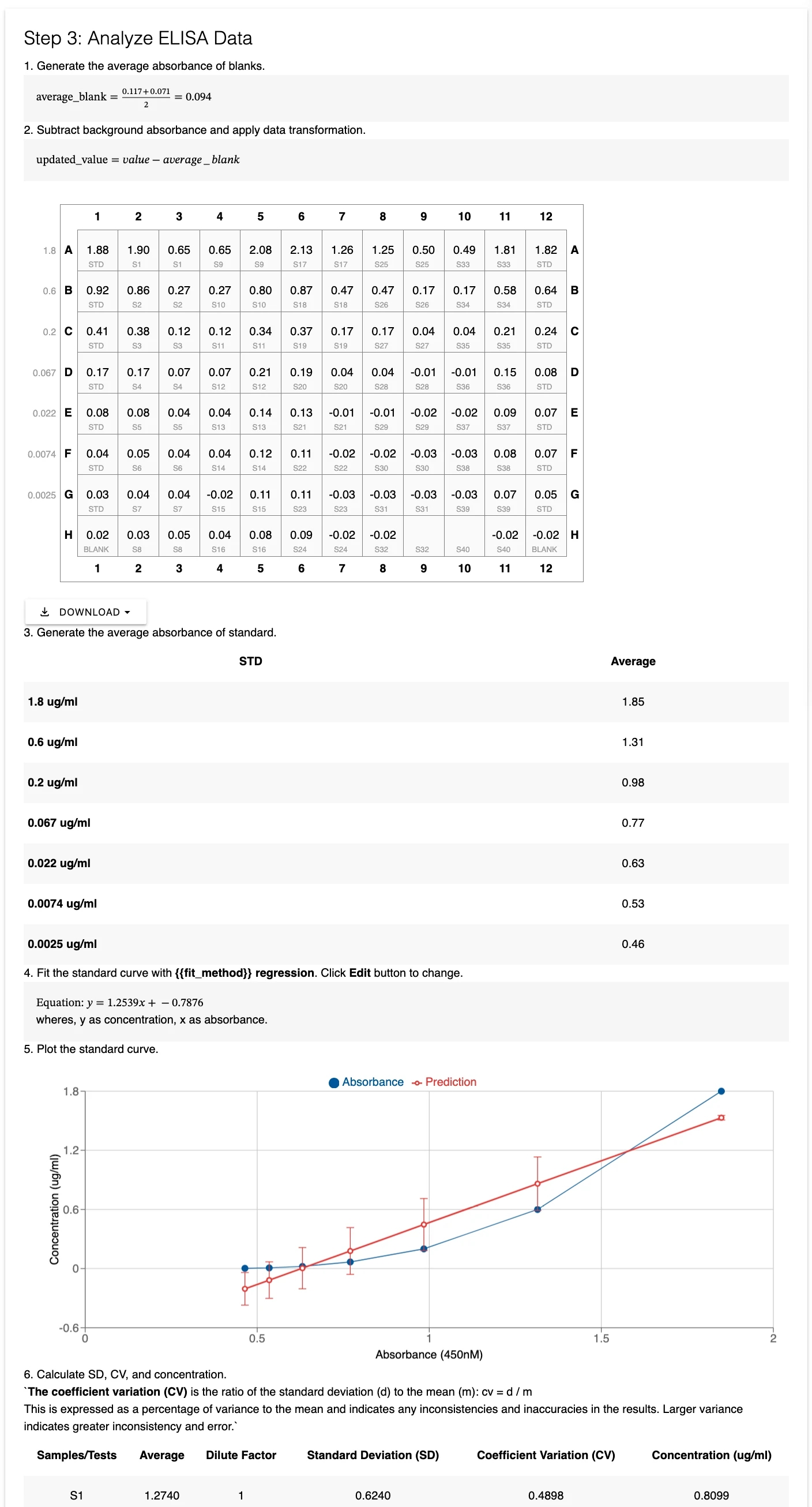
Key Features
Flexible Plate Layout:
Currently supports 96-well plates
Multiple input methods: manual entry, copy/paste from Excel, drag-and-drop file import
Automatic duplicate detection for replicate wells
Customizable dilution factors for each sample
Automated Analysis:
Automatic blank subtraction and normalization
Standard curve generation with multiple regression options
Sample concentration calculation with dilution correction
Quality metrics: SD, CV, R² values
Customizable analysis parameters
Data Visualization:
Standard curve plots with best-fit lines
Results tables with calculated concentrations
Quality metrics for assessing assay performance
Layout Setup Rules
When configuring your plate layout, follow these conventions:
First Cell: Enter concentration units (e.g., "ng/ml", "µg/ml", "pg/ml")
CONC Marker: Place "CONC" in the first column or row to designate standard concentrations
Standard Wells: Label standard wells with "STD"
Blank Wells: Label blank wells with "BLANK"
Samples: Use unique identifiers for unknown samples
Replicates: Duplicate well names are automatically treated as replicates
Exclusions: Leave wells blank to exclude from analysis
Regression Methods
Choose the appropriate regression method for your standard curve:
Linear: Best for narrow concentration ranges with linear response
Polynomial: For slightly curved standard curves
Logarithmic: For log-transformed data
Quality Metrics
The widget automatically calculates:
Standard Deviation (SD): Measures variability between replicates
Coefficient of Variation (CV): Expresses variability as a percentage (SD/mean × 100)
R² Value: Indicates goodness of fit for the standard curve
Blank Average: Mean absorbance of blank wells
For optimal results, aim for CV values below 10% for standards and below 20% for samples. R² values above 0.95 indicate a good standard curve fit.
Creating and Managing ELISA Protocols
Navigate to the protocol table from the side menu
Click + Add to create a new protocol
Fill in the protocol details:
Name: Clear protocol title (e.g., "Human IL-6 ELISA Protocol")
Status: Set to "Drafting" for new protocols
Description: Assay type, kit manufacturer, sensitivity range
Add detailed protocol steps including:
Reagent preparation and equilibration
Plate coating or pre-coated plate handling
Blocking procedures
Standard preparation and dilution scheme
Sample preparation requirements
Incubation times and temperatures
Washing procedures and buffer compositions
Detection system and substrate development
Plate reading parameters (wavelength, timing)
Attach supporting documents:
ELISA kit insert
Standard preparation worksheet
Validation data
Quality control acceptance criteria
Update the Status as the protocol progresses:
Drafting: Under development or optimization
Pending Review: Ready for validation
Finalized: Validated and approved for routine use
Archived: Superseded protocols
Managing ELISA Experiments in List View
Viewing and Filtering
Search experiments by name, date, or analyte
Filter by signature status to find unsigned experiments
Sort by start date to track experiment chronology
Use date range filters for specific timeframes
Create custom views for different assay types
Organizing Experiments
Tag experiments by project, analyte, or study phase
Group related experiments in projects
Archive completed experiments
Export data for external analysis or reporting
For comprehensive information about list view features, see list view documentation.
Advanced Features
Batch Analysis Across Experiments
Compare results across multiple ELISA runs:
Create a project to group related ELISA experiments
Add all related experiments to the project
Use the list view to filter experiments by project
Export concentration data from multiple experiments for statistical analysis
Quality Control Tracking
Monitor assay performance over time:
Include quality control samples in each ELISA run
Document control values in the results section
Track control values across experiments to detect drift or assay variability
Set up alerts for out-of-range control values
Custom Plate Layout Templates
Create reusable plate layouts for routine assays:
Set up a complete plate layout in an ELISA experiment
Duplicate the experiment for use as a template
For new experiments, copy the layout section from the template
Only update sample identifiers and data, keeping the standard layout consistent
Using templates ensures consistency across experiments and speeds up plate setup for routine assays.
Troubleshooting
Issue: Standard Curve Has Poor Fit (Low R²)
Symptoms: R² value below 0.95 or curve doesn't fit data points well
Solution:
Verify standard concentrations were prepared correctly
Check for pipetting errors or air bubbles in standards
Try different regression methods (e.g., 4PL instead of linear for sigmoidal curves)
Exclude outlier standard points if contamination is suspected
Consider repeating the assay if standards are compromised
Issue: High CV Values for Replicates
Symptoms: CV exceeds 10% for standards or 20% for samples
Solution:
Review pipetting technique for accuracy and precision
Check for air bubbles in wells that can affect readings
Verify plate reader is functioning properly and calibrated
Ensure adequate mixing of samples before plating
Consider excluding outlier replicates if technical error is identified
Issue: Sample Concentrations Outside Standard Range
Symptoms: Calculated concentrations exceed highest standard or fall below lowest standard
Solution:
For high concentrations, dilute samples further and repeat the assay
Update dilution factors in the widget and reanalyze
For low concentrations, consider using a more sensitive assay kit
Concentrate samples if possible (e.g., lyophilization, ultrafiltration)
Sample concentrations should fall within the linear range of the standard curve for accurate quantification.
Issue: Cannot Paste Data from Excel
Symptoms: Paste operation doesn't populate plate layout or data grid
Solution:
Ensure data is copied as tab-delimited format (standard Excel copy)
Check that data dimensions match the plate size (8 rows × 12 columns for 96-well)
Remove any formatting or special characters from Excel before copying
Try copying a smaller section of data and pasting incrementally
As an alternative, save as CSV and use drag-and-drop file import
Issue: Analysis Button Disabled
Symptoms: Cannot click Analyze button to perform calculations
Solution:
Verify that plate layout is completely configured with standards and concentrations
Ensure absorbance data has been entered in the data grid
Check that at least 3 standard concentrations are defined
Confirm that concentration units are specified in the first cell
Try refreshing the page if the button remains disabled
Electronic Signatures and Compliance
Electronic signatures certify the accuracy and completeness of ELISA experiments, ensuring data integrity and regulatory compliance.
Signing an ELISA Experiment
Complete all sections: overview, procedure, layout, data, and analysis
Review all results and quality metrics for accuracy
Navigate to the Signers tab or click Open Signers in the workflow
Add co-signers if required by your quality system
Click Sign and enter your password to authenticate
For detailed information about the signature process, see Signers.
Audit Trail Features
Labii automatically maintains comprehensive audit trails:
Version History: Track all changes to layouts, data, and analysis parameters (Versions)
Activity Log: Record all actions including data entry, analysis runs, and signatures (Activities)
Access Tracking: Monitor who viewed the experiment and when (Visitors)
Printing and Exporting
Open the ELISA experiment in detail view
Click the More menu (three dots) at the top right
Select Print from the dropdown
Review the print preview showing layout, data, analysis, and standard curve plot
Print to paper or save as PDF for archival, publication, or sharing
Learn more at Print.
Related Documentation
The Electronic Lab Notebook (ELN) for ELISA provides a comprehensive, integrated platform for ELISA data analysis. By combining plate layout configuration, data entry, automated standard curve analysis, and electronic signatures in a single application, researchers can efficiently conduct ELISA assays while maintaining complete audit trails and regulatory compliance. The specialized ELISA Standard Curve widget eliminates the need for multiple disconnected software tools, streamlining the workflow from experimental setup through data interpretation and compliant documentation for immunology research, diagnostic testing, pharmaceutical development, and quality control laboratorie
Last updated